Right to Trial & FDA Upgrade Act
right-to-trial, fda-upgrade, clinical-trials, drug-development, healthcare-reform, regulatory, patient-rights, open-data, decentralized-trials, fda-v2
Be it enacted by the Senate and House of Representatives of the United States of America in Congress assembled,
1 TITLE I: SHORT TITLE; PURPOSE; FINDINGS; DEFINITIONS
1.1 SEC. 101. SHORT TITLE
This Act may be cited as the “Right‑to‑Trial and FDA Upgrade Act.”
1.2 SEC. 102. PURPOSE
The purpose of this Act is to radically accelerate medical progress by:
- Establishing a right for all patients to participate in decentralized clinical trials for any condition.
- Creating a zero-knowledge, decentralized global protocol to function as a public utility that dramatically reduces the cost and time of pragmatic clinical trials.
- Enabling this protocol to aggregate private, individual, time-series health data and publish the results of causal inference analyses that show the change from baseline for any measured outcome after any intervention, tailored to the multiomic cohort most similar to an individual, while maintaining perfect participant anonymity.
- Providing the open, interoperable infrastructure upon which existing and future clinical trial platforms, electronic health record systems, and other health technologies can integrate and build, transforming the system from a series of disconnected data silos into a unified learning health ecosystem.
1.3 SEC. 103. FINDINGS
Congress finds the following:
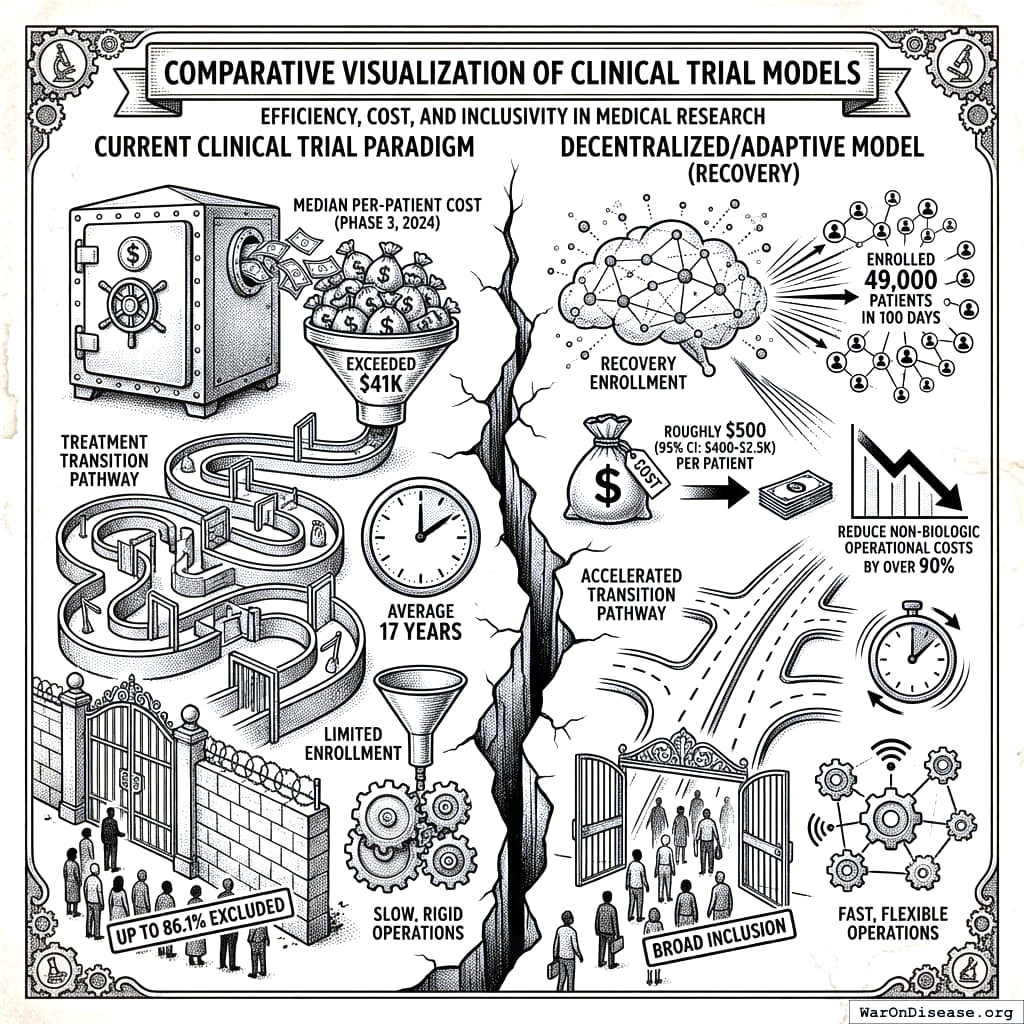
- New, effective treatments take an average of 17 years to transition from scientific discovery to clinical practice, a delay during which millions of patients suffer without access to potential cures.
- The current clinical trial paradigm is profoundly inefficient and exclusionary.
- Median per‑patient cost for a phase‑3 drug trial in 2024 exceeded $41K[2], inflating drug prices and limiting R‑&‑D on unpatentable therapies.
- Up to 86.1 percent of patients are excluded from participating in pivotal trials, limiting the generalizability of findings to real-world patient populations.
- The failure to publish negative results leads to redundant research, while rigid trial designs that cannot adapt to incoming data stifle innovation.
- The short duration of most trials results in a critical lack of data on the long-term safety and efficacy of an intervention.
- As a consequence of these systemic failures, an estimated 95 percent of rare diseases lack a single FDA-approved treatment, and effective therapies for common conditions remain undiscovered or inaccessible.
- The U.K. RECOVERY pragmatic trial enrolled 49,000 patients in 100 days at roughly $500[3], demonstrating that a decentralized, adaptive model can reduce the non-biologic operational costs of clinical research by over 90 percent through automation of data management, monitoring, and administrative functions.
- The strategic application of artificial intelligence in healthcare has the potential to yield substantial economic benefits, with studies indicating that AI could reduce national healthcare spending by 5 to 10 percent annually by optimizing diagnostics, personalizing treatments, and improving the efficiency of health-related research and development.
- Publicly financed, algorithm-targeted discounts on patient-borne trial participation costs, aimed at maximizing quality‑adjusted life‑years (QALYs) per federal dollar, can enhance access to trials, with patients covering the net costs of their participation. Funding for these subsidies can be sourced through innovative mechanisms like a decentralized institutes of health (DIH), a global treasury that raises capital by issuing bonds and is repaid by nations participating in a global health treaty.
- The establishment of a global health data protocol, analogous to the foundational protocols of the internet, is necessary to securely connect disparate data silos and accelerate medical research worldwide. The FDA.gov v2 Decentralized Health Protocol is the United States’ initial contribution to this protocol, a public utility providing the secure, open-source backend for a new generation of health innovation. It creates a global public good by enabling research on all promising therapies, especially those without commercial potential that are underserved by private-sector research.
- A transparent, open-access reference implementation for trial design, recruitment, data submission, and cost disclosure, as envisioned by the FDA.gov v2 Decentralized Health Protocol, creates a competitive environment among trial sponsors, pushing them to adopt cost-efficient methods and transparent pricing for trial operations.
- Modernizing FDA regulation to embrace real‑world evidence, remote monitoring, and validated non‑animal test methods accelerates safe cures.
- The Protocol serves not only the FDA but as shared, open-source public infrastructure for all federal, state, and international health authorities, creating a globally harmonized ecosystem for evidence generation and public health.
- This Act affirms the fundamental right of every person to access and participate in scientific research relevant to their health. By empowering individuals as both participants and trial creators, the United States can transform its regulatory infrastructure into a global public good, accelerating medical progress for all.
- The ultimate objective of the Protocol is to evolve into a fully decentralized and autonomous global protocol that is not reliant on the continued stewardship of any single government, corporate entity, or administrative body. The principle of progressive decentralization shall guide its development, ensuring that all governance and operational functions are systematically automated or migrated to secure, on-chain, community-ratified processes, thereby creating a resilient and permanently neutral public utility.
1.4 SEC. 104. DEFINITIONS
In this Act:
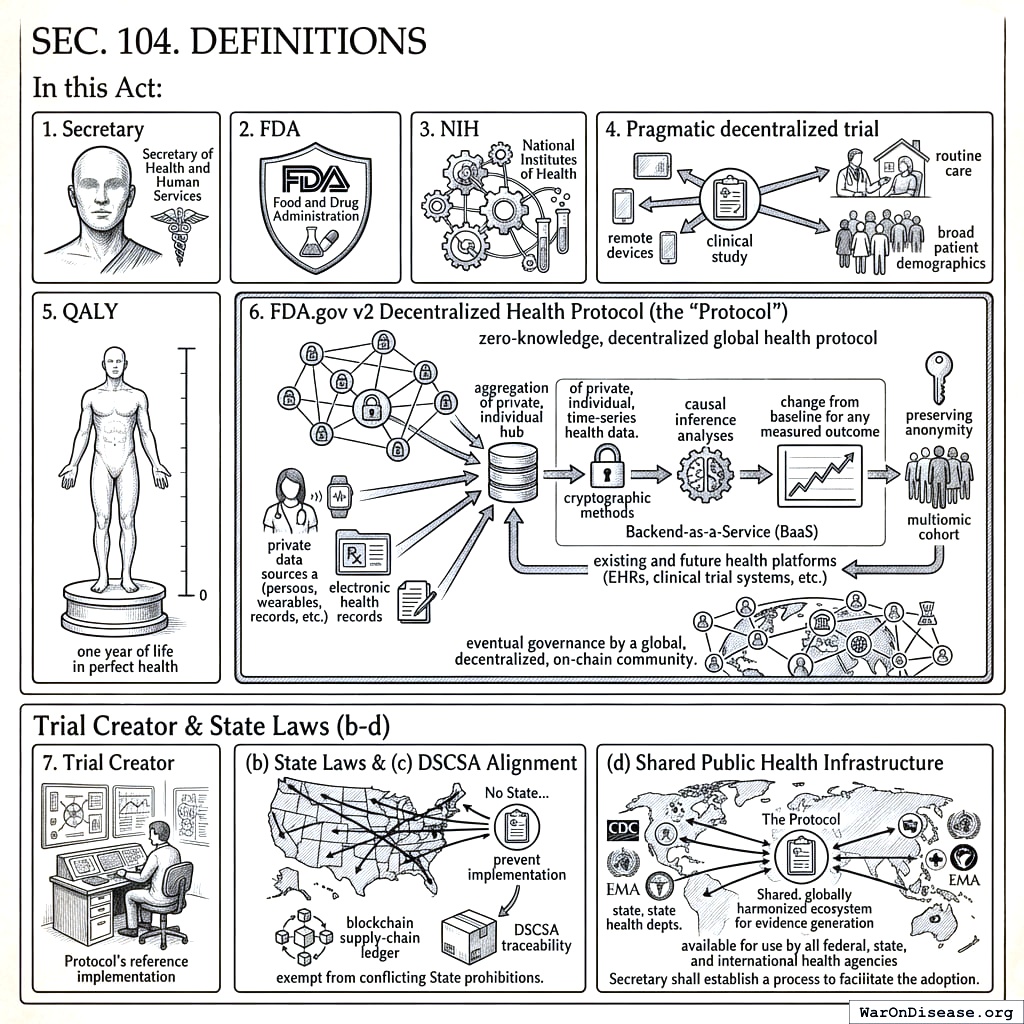
- Secretary means the Secretary of Health and Human Services.
- FDA means the Food and Drug Administration.
- NIH means the National Institutes of Health.
- Pragmatic decentralized trial means a clinical study integrated into routine care, allowing remote or local data capture, minimal exclusions, and broad patient demographics.
- QALY means a quality‑adjusted life‑year, one year of life in perfect health.
- FDA.gov v2 Decentralized Health Protocol (the “Protocol”) means the zero-knowledge, decentralized global health protocol initiated by the United States. Its primary function is to serve as a secure, open-source public utility that allows for the aggregation of private, individual, time-series health data. The Protocol is designed to perform and publish causal inference analyses on this aggregated data, showing the change from baseline for any measured outcome after any intervention for the multiomic cohort most similar to a given individual, while preserving the anonymity of all data contributors through cryptographic methods. It is intended to function as a Backend-as-a-Service (BaaS) with which existing and future health platforms (EHRs, clinical trial systems, etc.) can integrate, and is intended for eventual governance by a global, decentralized, on-chain community.
- Trial Creator means any individual, institution, or entity that designs, initiates, and manages a trial using the Protocol’s reference implementation.
- State Laws. No State or political subdivision may regulate the practice of tele‑medicine, pharmacy licensure, or shipment of investigational products in a manner that prevents implementation of this Act. Specifically, a licensed prescriber participating under an FDA‑approved protocol shall be deemed licensed in all States for the limited purpose of providing investigational treatment under this Act, and pharmacies dispensing or shipping such products pursuant to the blockchain supply‑chain ledger are exempt from conflicting State prohibitions.
- DSCSA Alignment. All investigational shipments must utilize the protocol’s ledger to satisfy DSCSA traceability.
- Shared Public Health Infrastructure. The Protocol shall be designed and maintained as extensible infrastructure available for use by all federal, state, and international health agencies. The Secretary shall establish a process to facilitate the adoption of the Protocol by other agencies for their own regulatory, research, and public health surveillance purposes, thereby creating a shared, globally harmonized ecosystem for evidence generation.
2 TITLE II: FDA Upgrade AND CLINICAL‑TRIAL INNOVATION
2.1 SEC. 201. ACCELERATED ADOPTION OF ALTERNATIVE PRECLINICAL TEST METHODS
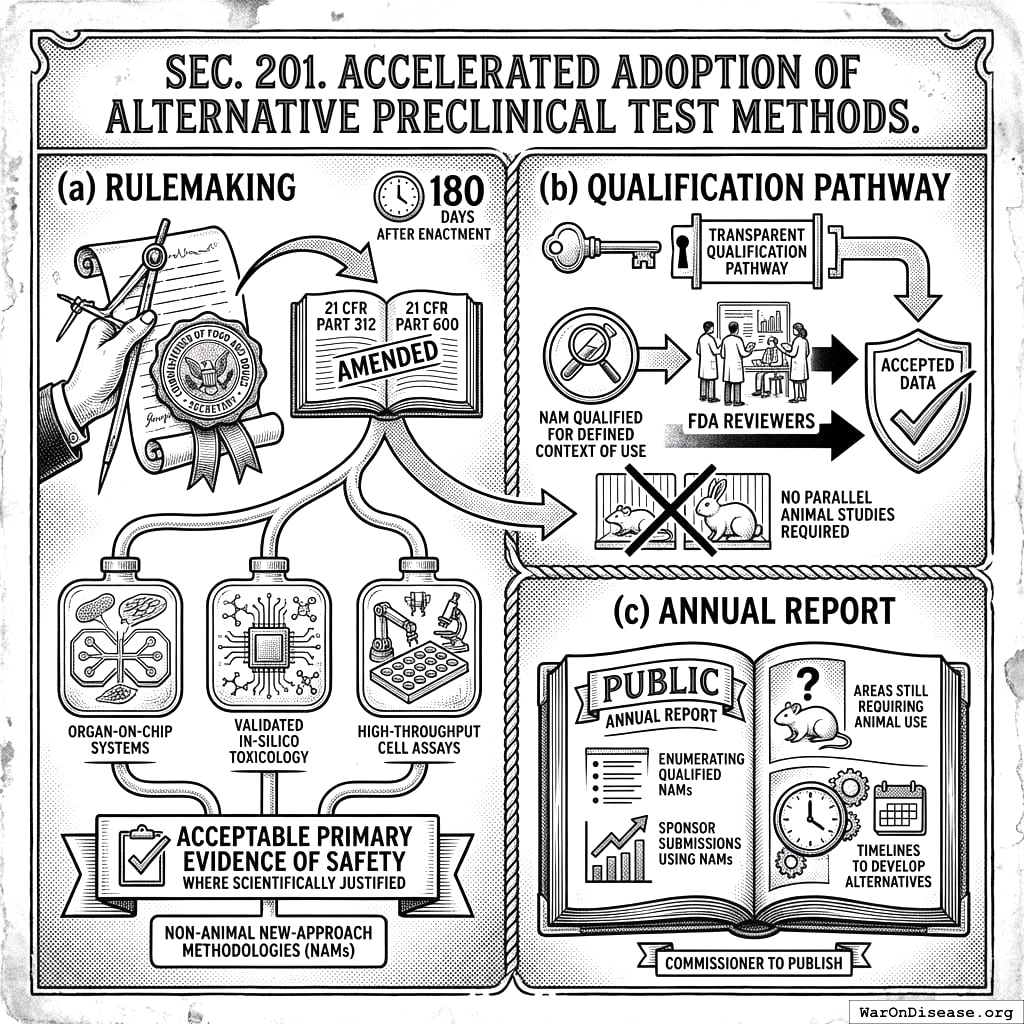
- Rulemaking. Not later than 180 days after enactment, the Secretary, acting through the Commissioner of Food and Drugs, shall issue final regulations amending 21 CFR Parts 312 and 600 to permit non‑animal New‑Approach Methodologies (NAMs), including organ‑on‑chip systems, validated in‑silico toxicology, and high‑throughput cell assays, as acceptable primary evidence of safety where scientifically justified.
- Qualification pathway. The regulations shall establish a transparent qualification pathway; once a NAM is qualified for a defined context of use, FDA reviewers shall accept data from that method without requiring parallel animal studies.
- Annual report. The Commissioner shall publish an annual public report enumerating qualified NAMs, sponsor submissions using NAMs, and areas still requiring animal use with timelines to develop alternatives.
2.2 SEC. 202. GUIDANCE ON DECENTRALISED, ADAPTIVE, AND REAL‑WORLD‑EVIDENCE TRIALS
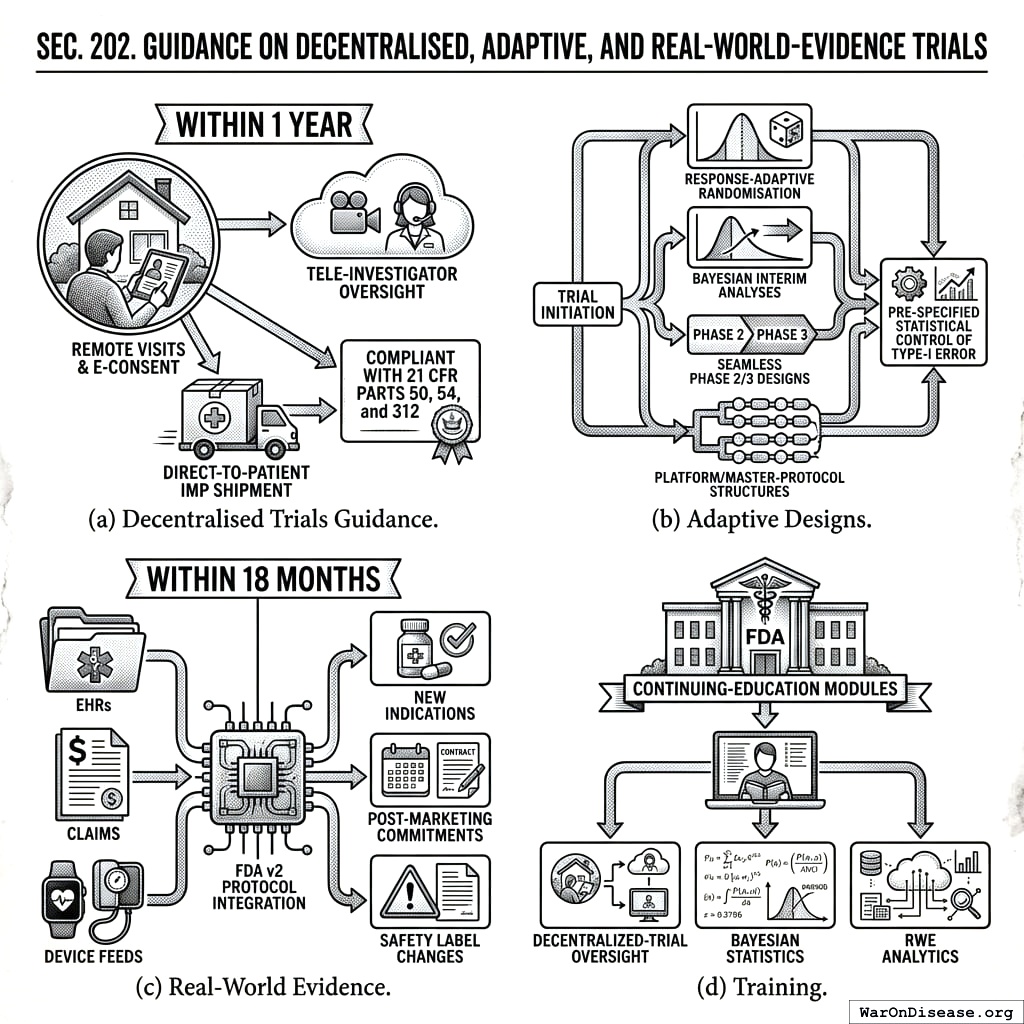
- Decentralised Trials Guidance. Within 1 year the Secretary shall issue final guidance recognising remote visits, tele‑investigator oversight, direct‑to‑patient IMP shipment, and e‑consent, as compliant with 21 CFR Parts 50, 54, and 312.
- Adaptive Designs. Guidance shall allow response‑adaptive randomisation, Bayesian interim analyses, seamless phase 2/3 designs, and platform/master‑protocol structures, provided pre‑specified statistical control of type‑I error.
- Real‑World Evidence. Within 18 months the Secretary shall publish a framework specifying how real‑world data (EHRs, claims, device feeds) integrated via the FDA v2 Protocol may support new indications, post‑marketing commitments, or safety label changes.
- Training. FDA shall establish continuing‑education modules to train reviewers in decentralized‑trial oversight, Bayesian statistics, and RWE analytics.
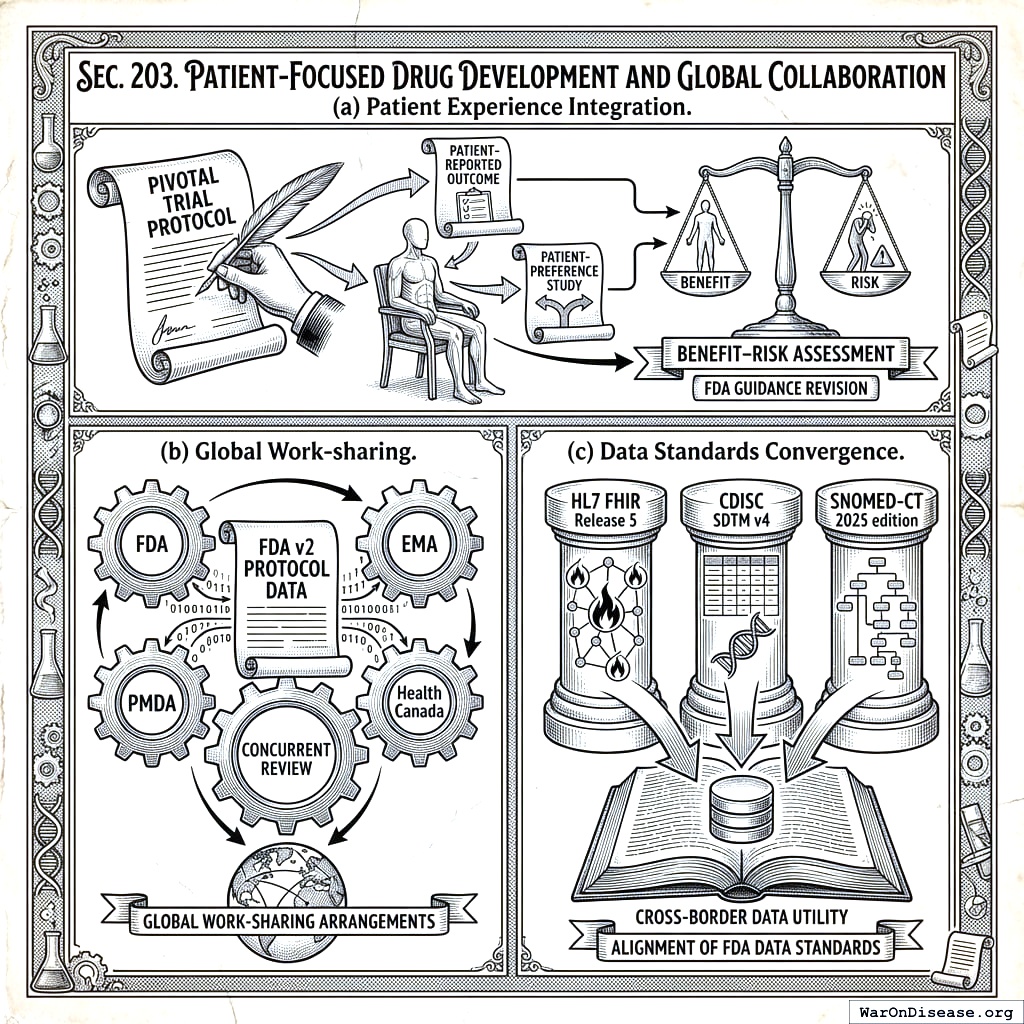
2.3 SEC. 203. PATIENT‑FOCUSED DRUG DEVELOPMENT AND GLOBAL COLLABORATION
- Patient Experience Integration. FDA shall revise Patient‑Focused Drug Development guidance to require that every pivotal trial protocol include at least one patient‑reported outcome or patient‑preference study relevant to benefit–risk assessment.
- Global Work‑sharing. The Secretary may enter into work‑sharing arrangements with peer regulators (EMA, PMDA, Health Canada) for concurrent review of applications utilising FDA v2 Protocol data.
- Data Standards Convergence. The Secretary shall align FDA data standards with HL7 FHIR Release 5, CDISC SDTM v4, and SNOMED‑CT 2025 edition to ensure cross‑border data utility.
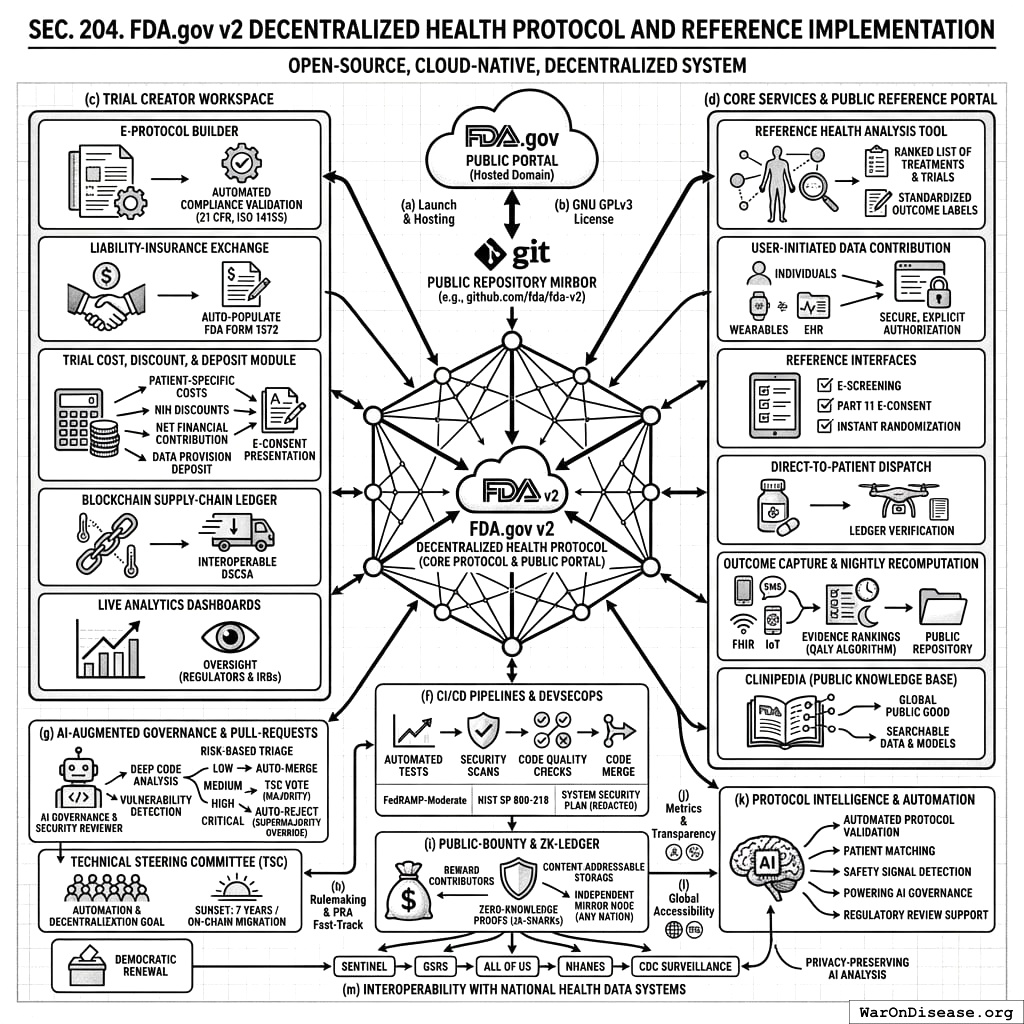
2.4 SEC. 204. FDA.gov v2 DECENTRALIZED HEALTH PROTOCOL AND REFERENCE IMPLEMENTATION
- Launch & Hosting. Within 12 months after enactment, the Secretary shall deploy an open-source, cloud-native FDA.gov v2 Decentralized Health Protocol, which shall include the FDA.gov Public Portal hosted on the fda.gov domain. All source code shall be mirrored in real-time to a public repository (e.g., github.com/fda/fda-v2).
- Mandatory Open‑Source Licence. All code shall be released under the GNU General Public License v3.0. Any proprietary dependency shall be replaced or dual‑licensed within 24 months.
- Trial Creator Workspace Functions. The FDA.gov Public Portal shall provide:
- E‑Protocol Builder with templates and automated compliance validation (21 CFR Parts 312/812, ISO 14155) that lets any Trial Creator, regardless of prior regulatory expertise, design and launch a study.
- Liability‑Insurance Exchange for real‑time per‑subject quotes; selections auto‑populate FDA Form 1572.
- Trial Cost, Discount, and Deposit Module facilitating: (A) the transparent calculation and disclosure of estimated patient-specific trial participation costs as provided by the trial sponsor; (B) the application of any applicable NIH-funded participation discounts (pursuant to SEC. 303); (C) the management of the net financial contribution required from the participant (as per SEC. 304(b)); and (D) the collection, holding, and refund of a patient data provision deposit (as per SEC. 304). All cost components, applicable discounts, the final net cost to the participant, and details of the data provision deposit shall be clearly itemized and presented to the participant during the e-consent process (as per SEC. 302(b) and SEC. 304).
- Blockchain Supply‑Chain Ledger[9] interoperable with DSCSA (§ 360eee‑3) to capture temperature, custody, delivery.
- Live Analytics Dashboards for enrolment, compliance, blinded efficacy; regulators & IRBs get read‑only oversight.
- Core Protocol Services and Public Reference Portal. The Protocol’s primary function is to provide core backend services accessible via its open API. To ensure baseline access for all citizens and to serve as a model for third-party developers, the Secretary shall host the public-facing FDA.gov Public Portal as part of the reference implementation. This framework shall:
- Provide a Reference Health Analysis Tool. The portal lets users connect their data to a secure reference agent that integrates and analyzes their health information to generate a ranked list of treatments & trials, presented alongside standardized “Outcome Labels.”
- Enable User-Initiated Data Contribution. The Protocol shall provide standardized tools (including APIs and SDKs) that let individuals securely contribute their health data from diverse sources. All data sharing shall be explicitly initiated and authorized by the user.
- Provide reference interfaces for single‑session e‑screening, Part 11 e‑consent, and instant randomisation for matched trials through the portal.
- Coordinate direct‑to‑patient or local‑pharmacy IMP dispatch with ledger verification.
- Capture outcomes via mobile app, SMS/IVR, FHIR push, and IoT feeds; data loop into evidence rankings which are recomputed nightly. The source code, feature weights, and a reproducible computational notebook for each annual release of the QALY‑ranking algorithm shall be posted in the public repository within 30 days of model deployment.
- Contribute to and draw from a Publicly Accessible Knowledge Base (dubbed “Clinipedia”) of all quantified food and drug effects, continuously updated with new data and analysis from the Protocol. This knowledge base, including all underlying data, analytical models, and evidence rankings, shall be made easily searchable and accessible to all, serving as a global public good.
- The Protocol’s Open API. The Protocol shall function as a Backend-as-a-Service (BaaS) for the national health technology ecosystem. Its primary deliverable is the secure, versioned, and documented open API. All de‑identified data shall be exposed through this RESTful API that is HL7 FHIR‑R5 compliant and meets 42 U.S.C. § 300jj‑52. The reference implementation is explicitly intended not to compete with consumer-facing applications but to provide the secure, interoperable rails upon which a competitive ecosystem of third-party tools and services can be built.
- Continuous Integration/Continuous Deployment (CI/CD). The Secretary shall maintain automated unit‑test, security‑scan, and code‑quality pipelines that must pass before any code merge. CI results shall be publicly viewable and the Protocol’s reference implementation shall maintain compliance with FedRAMP‑Moderate[6] and NIST SP 800‑218 DevSecOps guidelines; the System Security Plan and Authority‑to‑Operate letter shall be posted in redacted form.
- AI-Augmented Governance & Pull‑Request Acceptance.
- AI-Assisted Review. All pull requests (PRs) submitted to the public repository shall undergo an automated, comprehensive review by one or more designated AI Governance and Security Review systems. These systems shall be open-source and trained to perform deep code analysis, identify potential vulnerabilities, assess compliance with architectural standards, and model the risk of economic exploits.
- Risk-Based Triage. The AI Reviewer shall assign each PR a risk score (e.g., “Low,” “Medium,” “High,” “Critical”). This score determines the review and merge process:
- Low-Risk PRs: Shall be merged automatically within 72 hours if they pass all CI tests, unless a TSC member manually flags it for review.
- Medium-Risk PRs: Shall be automatically paused and require an explicit simple majority approval vote from the TSC for merger.
- High-Risk or Critical-Risk PRs: Shall be automatically rejected and may not be merged without a two-thirds supermajority vote from the TSC to override the AI’s finding, accompanied by a published justification.
- Technical Steering Committee (TSC). A nine‑member TSC is hereby established to oversee the repository, adjudicate flagged PRs, and manage the AI Governance Reviewers. The TSC is mandated to continuously pursue the automation and decentralization of its own functions, with the long-term goal of migrating governance decisions to a secure, on-chain, community-ratified process. To ensure this transition, the authority of the TSC as constituted herein shall sunset no later than 7 years after the date of enactment of this Act, or upon a determination by the Secretary that a secure and viable on-chain governance model is operational, whichever is earlier. The final act of the TSC shall be to ratify the migration to the successor governance protocol. Composition: 2 FDA officials, 1 NIH representative, 1 representative from a peer international regulator (e.g., EMA, PMDA), 1 patient‑advocacy representative, 1 open‑source AI/ML security expert elected by contributors, 1 biostatistician, 1 cyber‑security expert, and 1 industry sponsor representative. The Secretary shall pursue agreements to add representatives from at least two other international health authorities or bodies within 24 months.
- Appeal and Manual Override. Any contributor may appeal an AI rejection or TSC decision to the FDA Chief Scientist, who must respond within 30 days. The TSC retains the authority to manually re-classify any PR with a two-thirds vote.
- Democratic Renewal. The community-elected AI/ML security expert and patient-advocate seats are subject to annual election by contributors (defined as those with ≥5 merged PRs in the preceding year) using ranked‑choice voting via a transparent, verifiable online ballot.
- Rulemaking & PRA Fast‑Track. Within 180 days the Secretary shall issue interim final rules specifying technical standards for each module, standards for the content, format, and regular updating of Outcome Labels mandated under subsection (d)(1) of this section, codifying the TSC charter, and invoking 44 U.S.C. § 3507(h) such that any Information‑Collection Request[5] tied to the FDA v2 Protocol obtains OMB clearance within 60 days. Sponsors or investigators that fail to comply with these rules may be suspended under 21 U.S.C. § 331(f).
- Public‑Bounty & Zero‑Knowledge Ledger. The Secretary shall operate a continuous public bounty program, funded under § 402(a), to reward external contributors for merged pull‑requests, vulnerability disclosures, and feature enhancements. The AI Governance and Security Reviewer shall be used to automatically verify vulnerability submissions, score their severity, and recommend payment amounts to expedite the bounty process. Bounties shall be posted openly as issues in the public repository with dollar amounts and paid within 30 days of merge. Furthermore, all patient‑level data aggregated and analyzed by the Protocol shall be represented as zero‑knowledge proofs (e.g., zk‑SNARK commitments) and stored via content‑addressable storage, permitting any nation‑state or regional authority to run an independent mirror node and verify ledger integrity without accessing protected health information.
Metrics & Transparency. Annual public report: protocol uptime, median time‑to‑trial launch, pull‑request merge rate, unresolved PR backlog, bounty payouts, penetration‑test findings, insurance‑premium benchmarks, and user‑satisfaction scores. The Secretary shall commission an independent penetration test every fiscal year and publish an executive summary of findings.
Protocol Intelligence and Automation. The Protocol shall leverage artificial intelligence and machine learning capabilities to enhance its functionalities, including but not limited to: (1) assisting sponsors with automated protocol validation checks during e-protocol building; (2) improving the precision of matching patients to suitable trials based on their comprehensive health data; (3) augmenting the analysis of aggregated, de-identified data for early safety signal detection and pharmacovigilance; (4) powering the AI Governance and Security Reviewer for automated code review, vulnerability detection, and pull-request adjudication as specified in subsection (g); and (5) supporting regulatory staff with tools for efficient data review where appropriate. All such AI/ML systems shall be developed with robust validation, transparency in function, and operate under human oversight, particularly for critical decision support.
Global Accessibility. To facilitate global participation and data collection, the FDA.gov Public Portal’s user interfaces for patients and trial creators shall be made accessible in multiple languages.
Interoperability with National Health Data Systems. The Protocol shall be designed to complement and enhance existing government health initiatives. The Secretary shall establish data sharing and interoperability frameworks to connect the Protocol with key national health programs, including but not limited to: the FDA’s Sentinel Initiative and Global Substance Registry System (GSRS); the National Institutes of Health’s All of Us Research Program; the National Health and Nutrition Examination Survey (NHANES); and the Centers for Disease Control and Prevention’s public health surveillance systems. This integration shall leverage the Protocol’s AI capabilities to analyze data across systems, enhancing national safety monitoring, research, and public health response capabilities while adhering to strict privacy-preserving protocols.
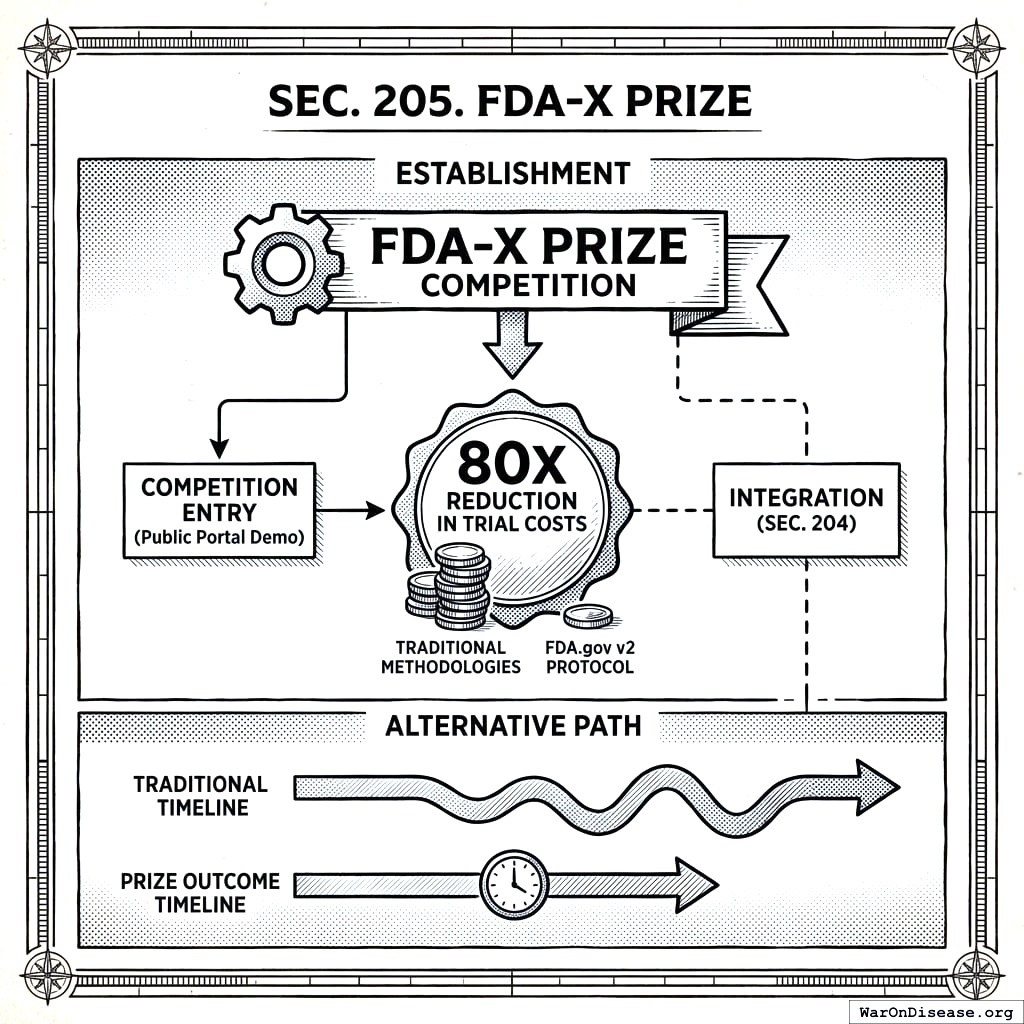
2.5 SEC. 205. FDA-X PRIZE
- Establishment. To accelerate the development of the FDA.gov v2 Protocol, the Secretary is authorized to establish an “FDA-X Prize” competition, with a prize purse to be determined by the Secretary, awarded to the entity that first develops and demonstrates a public portal meeting the core requirements outlined in Section 204 and which demonstrates the capacity to achieve at least an 80X reduction in per-patient trial costs compared to traditional methodologies.
- Alternative Development Path. The Secretary may use the prize competition as the primary mechanism for developing the protocol. In such a case, the timelines and responsibilities outlined in Section 204(a) shall be adjusted to reflect a successful prize outcome.
- Integration. If the prize is awarded for a platform that meets a substantial subset of requirements, the Secretary shall ensure its integration with any components developed or procured under Section 204.
3 TITLE III: UNIVERSAL TRIAL ACCESS (RIGHT‑TO‑TRIAL PROGRAM)
3.1 SEC. 301. UNIVERSAL ELIGIBILITY FOR INVESTIGATIONAL INTERVENTIONS
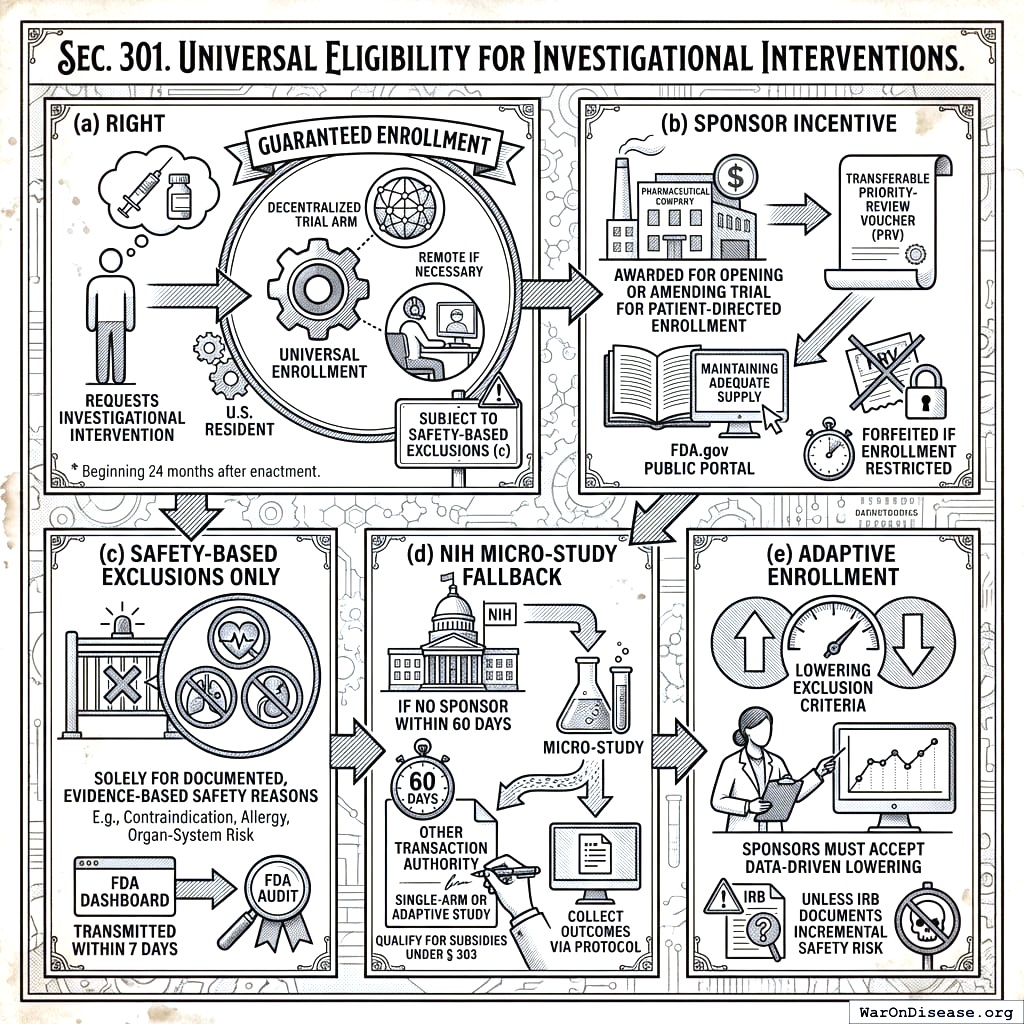
- Right. Beginning 24 months after enactment, any U.S. resident who requests an investigational intervention shall be guaranteed enrolment, remotely if necessary, in at least one pragmatic, decentralized trial arm evaluating that intervention, subject to the safety-based exclusions specified in subsection (c).
Sponsor Incentive. The Secretary shall award a transferable Priority‑Review Voucher (PRV) under section 524A of the Federal Food, Drug, and Cosmetic Act to any sponsor that, on or before the universal‑enrolment activation date, opens or amends a trial on the FDA.gov Public Portal to accept such patient‑directed enrolment and maintains adequate investigational‑product supply. A PRV is forfeited if the sponsor later restricts patient enrolment without a documented safety or manufacturing constraint.
Safety‑Based Exclusions Only. Sponsors or IRBs may exclude an individual patient solely for documented, evidence‑based safety reasons (e.g., a specific contraindication, allergy, or organ‑system risk) or if investigational‑product supply is demonstrably insufficient. Exclusion rationales must be transmitted to the FDA Dashboard within 7 days and are subject to FDA audit.
NIH Micro‑Study Fallback. If no sponsor operates an active investigational‑new‑drug application that can accept the patient within 60 days of request, the NIH, using Other Transaction Authority, shall initiate a single‑arm or adaptive micro‑study to provide the intervention under IND and collect outcomes via the Protocol; such micro‑studies qualify for subsidies under § 303.
Adaptive Enrolment. Sponsors participating under this section must accept data‑driven lowering of exclusion criteria unless an IRB documents incremental safety risk.
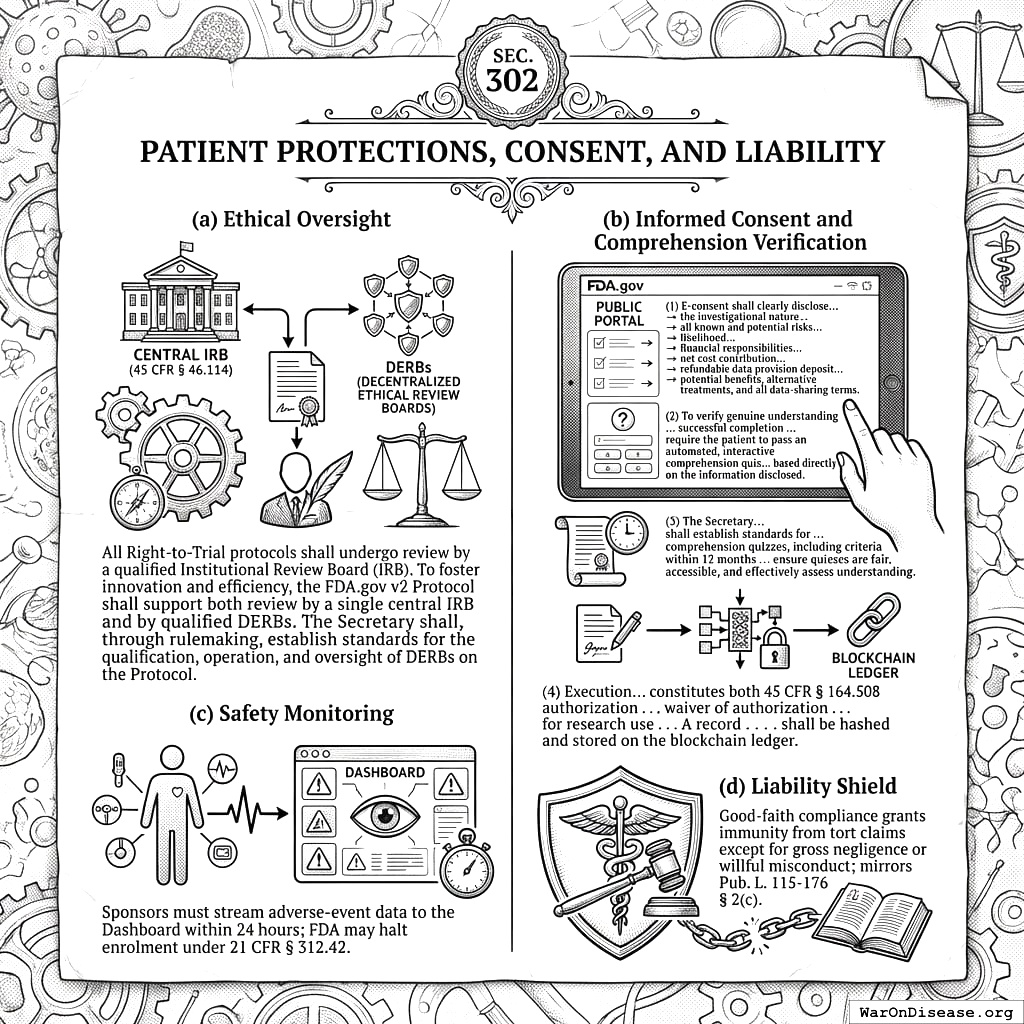
3.2 SEC. 302. PATIENT PROTECTIONS, CONSENT, AND LIABILITY
- Ethical Oversight. All Right‑to‑Trial protocols shall undergo review by a qualified Institutional Review Board (IRB). To foster innovation and efficiency, the FDA.gov v2 Protocol shall support both review by a single central IRB (per 45 CFR § 46.114[8]) and by qualified Decentralized Ethical Review Boards (DERBs). The Secretary shall, through rulemaking, establish standards for the qualification, operation, and oversight of DERBs on the Protocol.
- Informed Consent and Comprehension Verification.
- E-consent via the FDA.gov Public Portal shall clearly disclose, in an accessible format, the investigational nature of the trial, all known and potential risks including their likelihood based on available data (with particular emphasis on the most serious risks), the full details of patient financial responsibilities including the net cost contribution and the refundable data provision deposit (as detailed in SEC. 304), potential benefits, alternative treatments, and all data-sharing terms.
- To verify genuine understanding of the disclosed information, particularly concerning the most serious risks and their likelihood, successful completion of the e-consent process shall require the patient to pass an automated, interactive comprehension quiz administered through the FDA.gov Public Portal. This quiz shall be based directly on the information disclosed pursuant to paragraph (1) of this subsection.
- The Secretary, through rulemaking within 12 months of enactment, shall establish standards for the development, content, validation, and administration of such comprehension quizzes, including criteria for successful completion and procedures for patients who do not initially pass. These standards shall ensure quizzes are fair, accessible, and effectively assess understanding of critical information.
- Execution of the e-consent, following successful completion of the comprehension quiz, constitutes both 45 CFR § 164.508 authorization and, where applicable, a waiver of authorization under § 164.512(i)[11] for research use of protected health information, as approved by the reviewing IRB. A record of successful quiz completion and the signed consent shall be hashed and stored on the blockchain ledger.
- Safety Monitoring. Sponsors must stream adverse‑event data to the Dashboard within 24 hours; FDA may halt enrolment under 21 CFR § 312.42.
- Liability Shield. Good‑faith compliance grants immunity from tort claims except for gross negligence or willful misconduct; mirrors Pub. L. 115‑176 § 2(c).
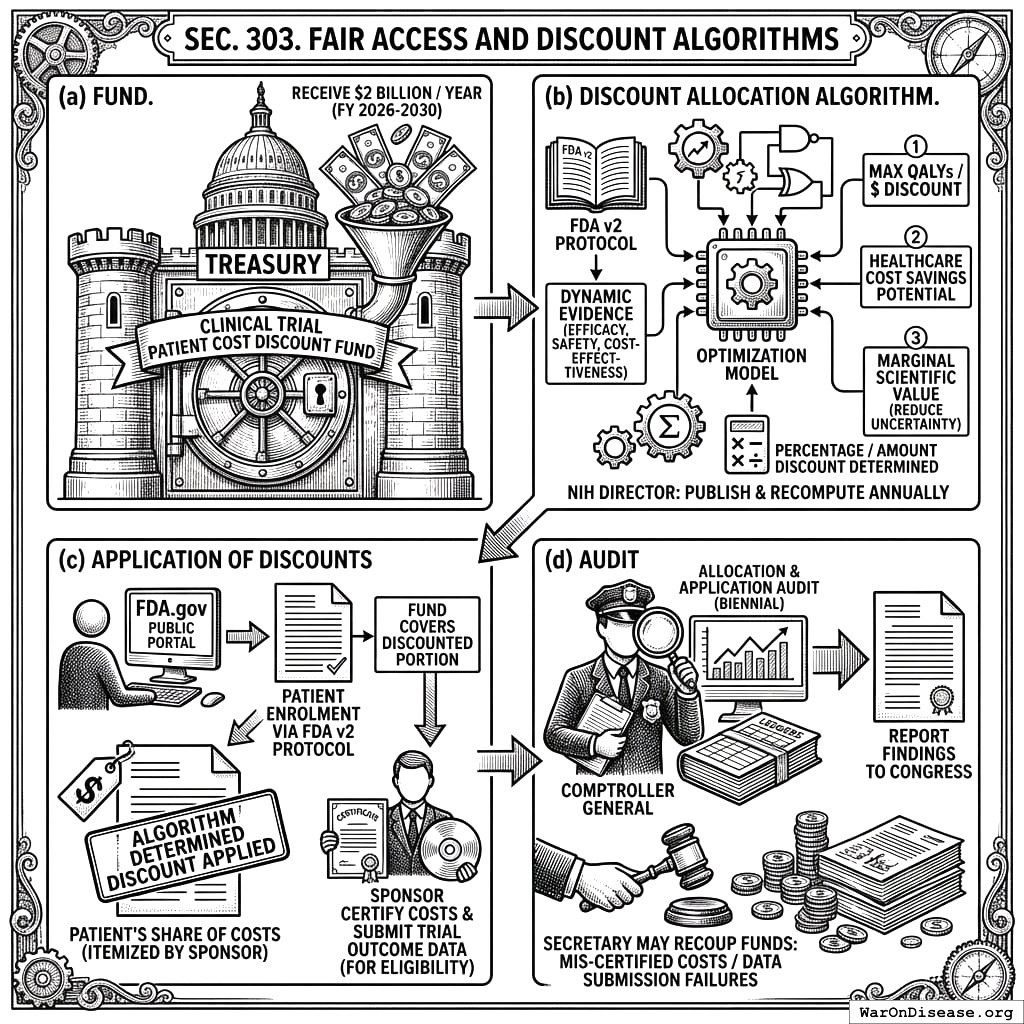
3.3 SEC. 303. FAIR ACCESS AND DISCOUNT ALGORITHMS
- Fund. A revolving Clinical Trial Patient Cost Discount Fund is hereby established within the Treasury, authorized to receive $2 billion for each of fiscal years 2026 through 2030.
Discount Allocation Algorithm. The NIH Director shall develop, publish, and annually recompute a transparent, open-source optimisation model for the allocation of discounts from the Fund. This model shall determine the percentage or amount of discount applicable to patient-borne costs for participation in specific trials available on the FDA v2 Protocol. The primary objectives for discount allocation shall be: (1) maximizing projected quality-adjusted life-years (QALYs) gained per dollar of discount provided; (2) the potential for significant healthcare system cost savings attributable to the research; and (3) the marginal scientific value of the pragmatic clinical trial, including its potential to reduce uncertainty for interventions with limited existing evidence but high potential impact. The algorithm shall dynamically incorporate new evidence on treatment efficacy, safety, and cost-effectiveness generated through the FDA v2 Protocol.
Application of Discounts. Upon a patient’s enrolment in a trial via the FDA v2 Protocol, the NIH shall authorize the application of the algorithmically determined discount to the patient’s share of trial participation costs, as itemized by the sponsor and displayed on the FDA.gov Public Portal (per SEC. 204(c)(3) and SEC. 304). The Fund shall cover the discounted portion of such costs. Sponsors shall certify the full, itemized costs of participation and submit all required trial outcome data for continued eligibility to have their trials included in the discount program.
Audit. The Comptroller General of the United States shall audit the allocation and application of discounts from the Fund biennially and report findings to Congress. The Secretary may recoup any funds associated with discounts applied to mis-certified costs or for trials not meeting data submission requirements.
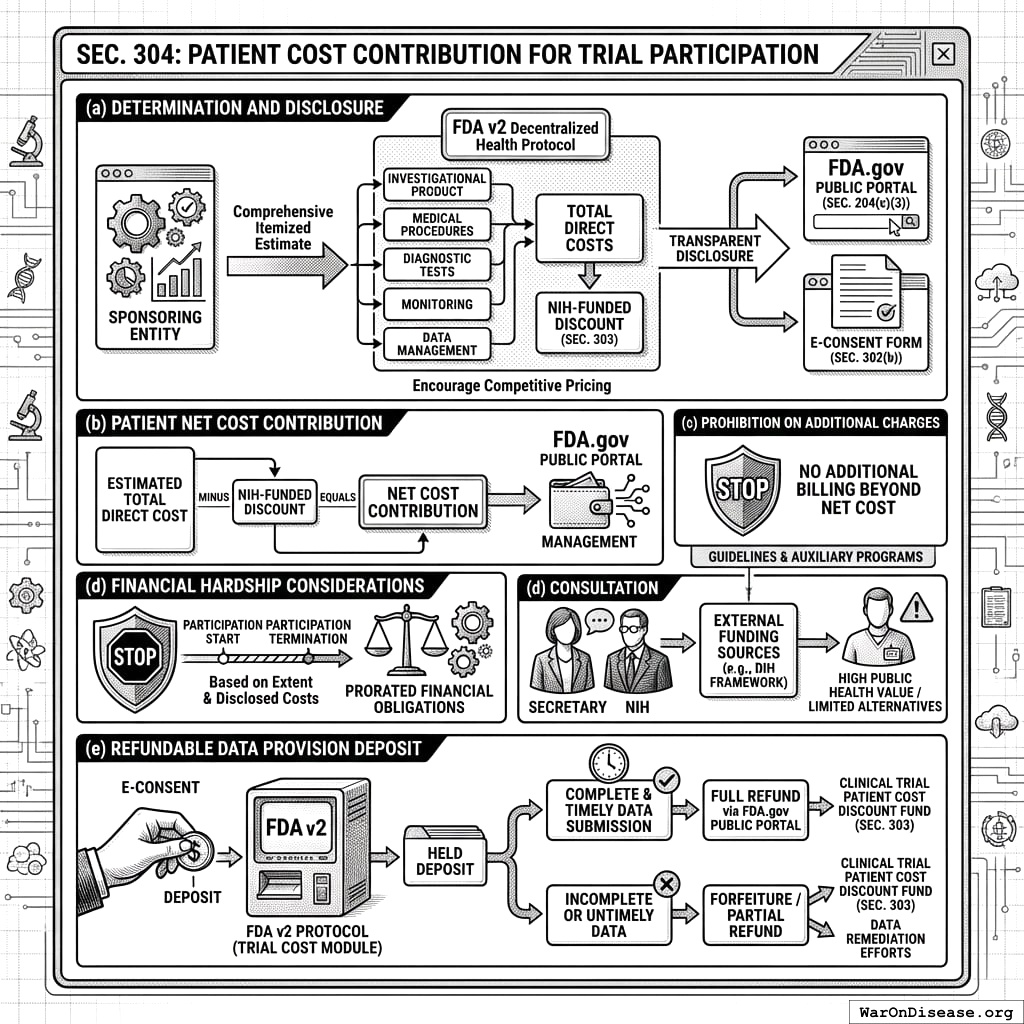
3.4 SEC. 304. PATIENT COST CONTRIBUTION FOR TRIAL PARTICIPATION
- Determination and Disclosure of Participation Costs. Prior to a patient consenting to a trial, the sponsoring entity shall determine and provide to the FDA v2 Decentralized Health Protocol a comprehensive, itemized estimate of all direct costs associated with that specific patient’s anticipated participation in the trial. This shall include, but not be limited to, costs of investigational product, necessary medical procedures, diagnostic tests, monitoring, and data management directly attributable to the research participant. These estimated costs, alongside the NIH-funded discount applicable under SEC. 303, and the final net cost to the patient, shall be transparently displayed to the potential participant via the FDA.gov Public Portal (SEC. 204(c)(3)) and detailed in the e-consent form (SEC. 302(b)). This transparent disclosure of itemized costs on a decentralized framework is intended to encourage competitive and efficient pricing by sponsoring entities.
- Patient Net Cost Contribution. Each participant shall be responsible for contributing the net cost of their trial participation, calculated as the estimated total direct cost of participation (as determined in subsection (a) of this section) minus the NIH-funded discount (as determined and applied under SEC. 303). The FDA.gov Public Portal shall facilitate the management of this net cost contribution.
- Prohibition on Additional Charges. Sponsors or investigating sites may not bill or charge a participant for any costs related to their trial participation beyond the final net cost contribution calculated and disclosed through the FDA v2 Protocol as per subsections (a) and (b) of this section. Participants shall retain the right to terminate their participation in the trial at any time, subject to standard clinical and ethical procedures; financial obligations will be prorated based on the extent of participation and disclosed costs.
- Financial Hardship Considerations. The Secretary, in consultation with NIH, may establish guidelines or auxiliary programs to address financial hardship for patients for whom the net cost contribution, even after applicable discounts, remains a significant barrier to accessing trials deemed of high public health value or for conditions with limited alternative treatments. Such guidelines shall not obligate sponsors to cover these costs unless through separate, voluntary programs, but may coordinate with external funding sources, such as those that might be allocated through a decentralized institutes of health (DIH) framework.
- Refundable Data Provision Deposit.
- To incentivize the complete and timely submission of all data required by the trial protocol, each participant shall provide a refundable data provision deposit. The amount of such deposit shall be established by the Secretary through rulemaking, considering factors such as trial duration, data complexity, and the need to avoid undue financial burden on participants.
- The deposit shall be collected by the FDA v2 Protocol’s Trial Cost, Discount, and Deposit Module (as per SEC. 204(c)(3)) at the time of e-consent.
- The full deposit shall be refunded to the participant via the FDA.gov Public Portal upon certification by the trial sponsor or a designated Platform administrator that the participant has provided all protocol-required data within the specified timeframes.
- Conditions for partial refund or forfeiture of the deposit due to incomplete or untimely data submission shall be detailed in the e-consent form (SEC. 302(b)) and established by the Secretary through rulemaking. Forfeited deposits may be directed to the Clinical Trial Patient Cost Discount Fund (SEC. 303) or used to offset costs incurred by data remediation efforts.
4 TITLE IV: GENERAL PROVISIONS
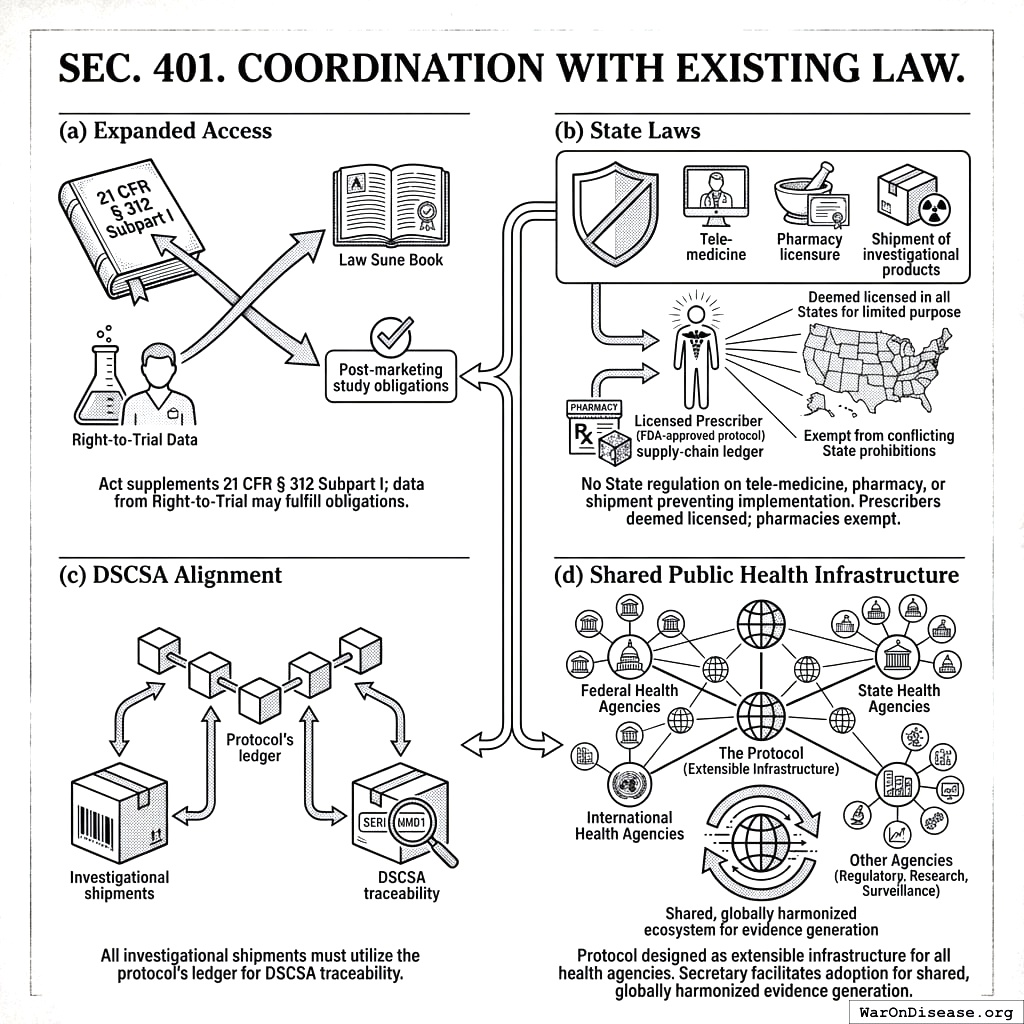
4.1 SEC. 401. COORDINATION WITH EXISTING LAW
- Expanded Access. This Act supplements 21 CFR § 312 Subpart I; data from Right‑to‑Trial may fulfill post‑marketing study obligations.
- State Laws. No State or political subdivision may regulate the practice of tele‑medicine, pharmacy licensure, or shipment of investigational products in a manner that prevents implementation of this Act. Specifically, a licensed prescriber participating under an FDA‑approved protocol shall be deemed licensed in all States for the limited purpose of providing investigational treatment under this Act, and pharmacies dispensing or shipping such products pursuant to the blockchain supply‑chain ledger are exempt from conflicting State prohibitions.
- DSCSA Alignment. All investigational shipments must utilize the protocol’s ledger to satisfy DSCSA traceability.
- Shared Public Health Infrastructure. The Protocol shall be designed and maintained as extensible infrastructure available for use by all federal, state, and international health agencies. The Secretary shall establish a process to facilitate the adoption of the Protocol by other agencies for their own regulatory, research, and public health surveillance purposes, thereby creating a shared, globally harmonized ecosystem for evidence generation.
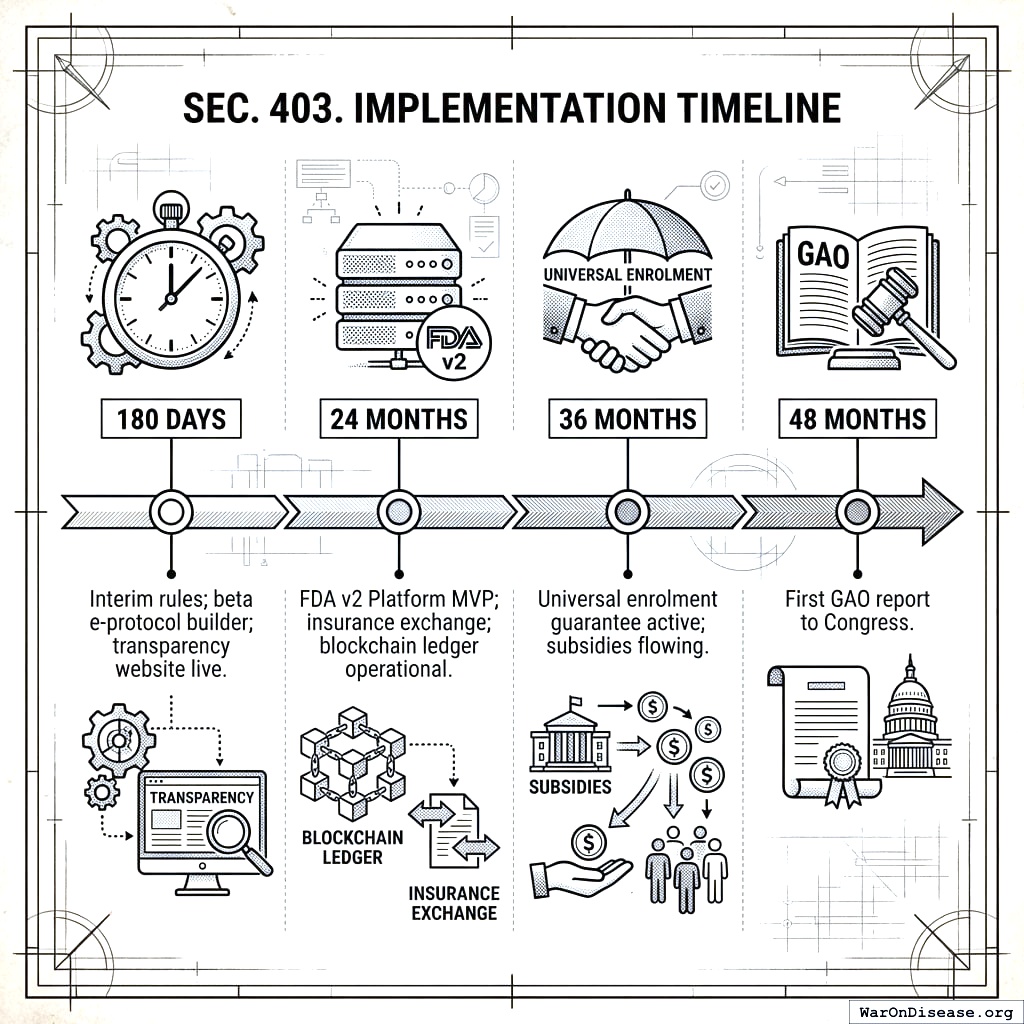
4.3 SEC. 403. IMPLEMENTATION TIMELINE
- 180 days: Interim rules; beta e‑protocol builder; transparency website live.
- 24 months: FDA v2 Platform MVP; insurance exchange; blockchain ledger operational.
- 36 months: Universal enrolment guarantee active; subsidies flowing.
- 48 months: First GAO report to Congress.
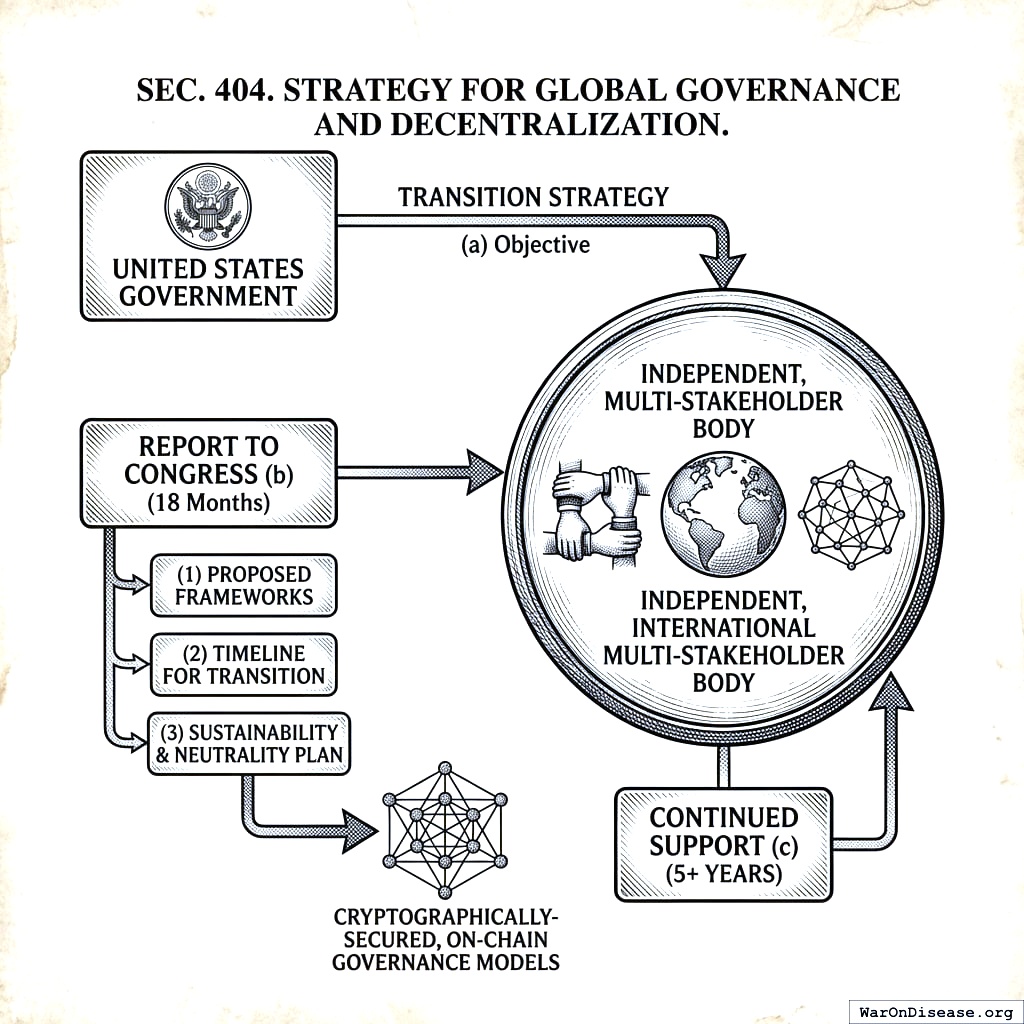
4.4 SEC. 404. STRATEGY FOR GLOBAL GOVERNANCE AND DECENTRALIZATION
- Objective. The Secretary, in coordination with the Secretary of State, shall develop and execute a strategy to transition the governance of the Protocol from the United States government to an independent, international multi-stakeholder body.
- Report to Congress. Within 18 months of enactment, the Secretary shall submit a report to Congress outlining this strategy, including: (1) proposed frameworks for a new international governance body; (2) a timeline for transitioning technical and administrative oversight; and (3) a plan for ensuring the long-term sustainability and neutrality of the protocol, including research into and pathways toward cryptographically-secured, on-chain governance models that reduce reliance on a central administrative body.
- Continued Support. The United States shall continue to provide technical and financial support for the Protocol during a transition period of no less than 5 years after an international governance body is established.
4.5 SEC. 405. SEVERABILITY
If any provision of this Act is held invalid, the remainder shall remain in effect.
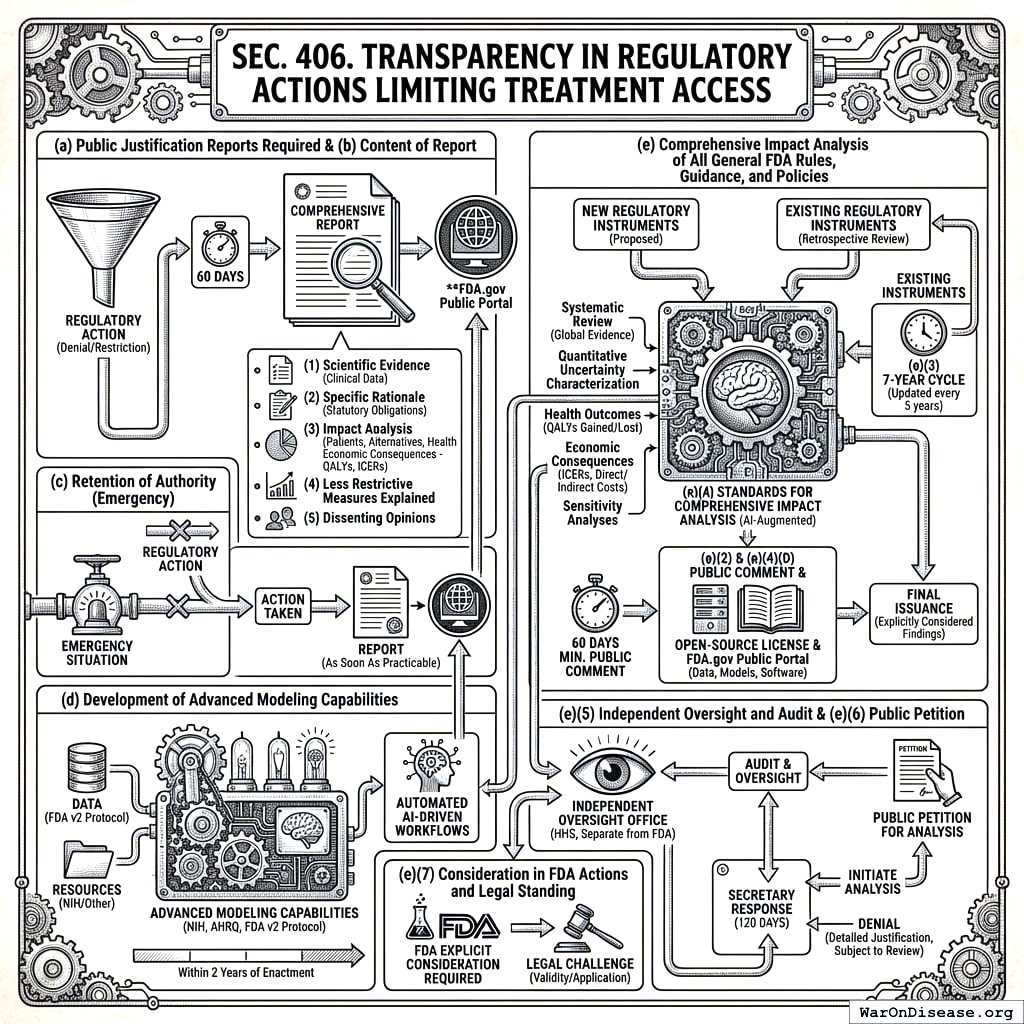
4.6 SEC. 406. TRANSPARENCY IN REGULATORY ACTIONS LIMITING TREATMENT ACCESS
- Public Justification Reports Required. For any regulatory action taken by the Secretary that denies, restricts, or withdraws patient access to any potential treatment, the Secretary shall, within 60 days of such action, publish a comprehensive report. This report shall be publicly available on the FDA.gov Public Portal.
Content of Report. Such report shall include, at a minimum: (1) a detailed summary of the scientific evidence regarding the treatment’s benefits and risks considered by the Secretary, including available clinical endpoint data; (2) the specific rationale for the regulatory action, including any statutory obligations influencing the decision; (3) an analysis of the anticipated impact of the action on patient populations, including consideration of available alternatives, unmet medical needs, and a summary of available information on potential health economic consequences, including, where feasible and appropriate, a qualitative or quantitative assessment of costs and benefits; provided that, upon certification by the Secretary that the capabilities developed under subsection (d) are sufficiently mature, such assessment shall include a quantitative health and economic modeling simulation as detailed therein; (4) an explanation of why less restrictive regulatory measures, if applicable, were deemed insufficient; and (5) any dissenting opinions from within the review team or advisory committees, if applicable.
Retention of Authority. Nothing in this section shall be construed to limit the Secretary’s authority to take necessary regulatory action to protect public health, including in emergency situations. In such cases, the report shall be published as soon as practicable following the action.
Development of Advanced Modeling Capabilities for Treatment Access Reports. The Secretary, in consultation with the Director of the NIH and the Director of the Agency for Healthcare Research Quality (AHRQ), shall, within 2 years of enactment, establish and ensure the operational maturity of robust capabilities for conducting the quantitative health and economic modeling simulations necessary to fulfill the reporting requirements under subsection (b) of this section. This shall include utilizing data from the FDA v2 Protocol, defining appropriate resource allocation from the NIH and other sources, and developing automated AI-driven workflows where feasible.
Comprehensive Impact Analysis of All General FDA Rules, Guidance, and Policies.
- Mandate for Analysis: To ensure all Food and Drug Administration (FDA) activities are demonstrably in the public interest and to quantify their effects on public health and the economy, the Secretary shall ensure that every proposed and existing FDA regulation (as defined in 21 C.F.R. Part 10), formal guidance document, and other generally applicable policy statement (hereinafter collectively referred to as “regulatory instruments”) undergoes a comprehensive, quantitative health and economic impact analysis as specified in this subsection.
- Prospective Analysis of New Regulatory Instruments: Except as provided in subsection (c) of this section for emergency actions, no new FDA regulatory instrument shall be finalized, issued, or take effect until a comprehensive impact analysis, meeting the requirements of paragraph (e)(4) of this subsection, has been completed, made public on the FDA.gov Public Portal for a period of no less than 60 days for public comment, and its findings explicitly considered and addressed by the Secretary in the final issuance. For emergency actions, the analysis shall be completed and published within 90 days of the instrument taking effect.
- Retrospective Analysis and Review of Existing Regulatory Instruments: The Secretary shall, within 1 year of enactment, establish and publish a prioritized schedule for the systematic review and comprehensive impact analysis of all significant existing FDA regulatory instruments. This schedule shall ensure that all such instruments are analyzed within 7 years of enactment. All analyses conducted under this subsection shall be updated at least every 5 years, or more frequently if significant new evidence or modeling capabilities emerge.
- Standards for Comprehensive Impact Analysis: Each analysis conducted under this subsection (e) shall:
- Be supported by dedicated resources, including a prespecified minimum percentage of the annual budget of the National Institutes of Health, to ensure its capacity, independence, and timeliness.
- Be based on a systematic review of all available global evidence, incorporate rigorous quantitative uncertainty characterization, and calculate projected individual and population-level health outcomes (including, but not limited to, quality-adjusted life-years (QALYs) gained or lost) and economic consequences (including, but not limited to, Incremental Cost-Effectiveness Ratios (ICERs) and direct and indirect costs to patients, the healthcare system, and society). Sensitivity analyses shall be conducted for all key assumptions.
- Be developed and executed primarily by advanced autonomous AI systems to ensure speed, consistency, and comprehensiveness. The role of human personnel shall be focused on oversight, final review, and the adjudication of complex edge cases flagged by the AI. All software, algorithms, data inputs, and models developed or utilized for these analyses shall be released under an open-source licence approved by the Open Source Initiative, published on the FDA.gov Public Portal, and subject to mechanisms that facilitate public inspection, contribution, and collaborative improvement, consistent with the AI-augmented governance principles outlined in SEC. 204(g);
- Be made publicly available in its entirety, including all underlying data, assumptions, and models, on the FDA.gov Public Portal in a user-friendly and accessible format.
- Independent Oversight and Audit: An independent office within the Department of Health and Human Services, separate from the Food and Drug Administration, shall be established or designated to oversee the methodologies, execution, and audit of analyses conducted under this subsection (e) to ensure objectivity and scientific integrity.
- Public Petition for Analysis: Any member of the public may petition the Secretary for a comprehensive impact analysis of any specific FDA regulatory instrument not yet analyzed or not recently updated. The Secretary shall respond to such petitions within 120 days, either by initiating the analysis or by publishing a detailed justification for denial, which shall itself be subject to review by the independent office established under paragraph (e)(5).
- Consideration in FDA Actions and Legal Standing: The FDA shall be required to explicitly consider and publicly respond to the findings of these impact analyses in all subsequent rulemaking, policy development, enforcement activities, and in the review of existing regulatory instruments. Failure to conduct or appropriately consider such analyses as mandated herein shall be grounds for legal challenge to the validity or application of the regulatory instrument.
4.7 REFERENCES
[1] ClinicalTrials.gov FY 2024 Annual Report, Table 4 (trial enrollment). [2] Moore, T. J., Zhang, H., Anderson, G., & Alexander, G. C. (2018). Estimated Costs of Pivotal Trials for Novel Therapeutic Agents Approved by the US Food and Drug Administration, 2015-2016. JAMA Internal Medicine, 178(11), 1451–1457. [3] See Decentralized Pragmatic Trials (RECOVERY) analysis, citing UKRI, Manhattan Institute, and others. [4] openFDA GitHub Repository, https://github.com/FDA. [5] 44 U.S.C. § 3507(h) fast‑track provision; Administrative Conference PRA Study (2012). [6] FedRAMP FAQ, ‘Understanding Baselines & Impact Levels’ (2024). [7] 42 CFR § 1001.952(bb) (value‑based safe‑harbour). [8] 45 CFR § 46.114, NIH Single‑IRB Policy (updated 2023). [9] FDA DSCSA Pilot Project Program – Final Report (2024). [10] U.S. Digital Service, Digital Service Playbook (2025). [11] 45 CFR § 164.512(i)(1) (HIPAA research waiver).